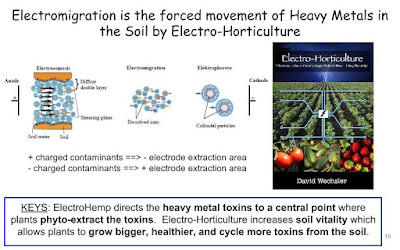
The study of heavy metal tolerance in plants in the late 1980s. The discovery of hyperaccumulator plants, which contain levels of heavy metals that would be highly toxic to other plants, prompted the idea of using certain plant species to extract metals from the soil and, in the process, clean up soil for other less tolerant plants.
 Scientists also found that certain plants could degrade organic contaminants by absorbing them from the soil and metabolizing them into less harmful chemicals.
Scientists also found that certain plants could degrade organic contaminants by absorbing them from the soil and metabolizing them into less harmful chemicals.
 In addition to plants, microorganisms that live in the rhizosphere (the actively growing root zone of the soil) play a major role in degrading organic chemicals, often using these chemicals as a carbon source in their metabolism. In many cases, even the physical presence of a plant can improve the condition of the soil, giving it structure and stability and altering hydrology by enhancing water retention and preventing erosion.
In addition to plants, microorganisms that live in the rhizosphere (the actively growing root zone of the soil) play a major role in degrading organic chemicals, often using these chemicals as a carbon source in their metabolism. In many cases, even the physical presence of a plant can improve the condition of the soil, giving it structure and stability and altering hydrology by enhancing water retention and preventing erosion.

There is no doubt that plants and the microbes associated with them can profoundly alter an ecosystem. Different types of phytoremediation have different potential results, such as accumulation of heavy metals in specific plant organs, voltilization from leaf surfaces, alteration of the form or availability of an organic chemical in the soil or within the plant, or actively excluding chemicals from plant tissues and keeping them out of the food chain.

The result depends on site-specific research and this approach is not generally appropriate for grossly contaminated soils that are an immediate ecological health risk. The major challenge to using phytoremediation effectively remains gaining an understanding of these various plant–chemical interactions and learning how to apply them safely in remediation programs
 phytoremediation science paper source
phytoremediation science paper source