The following Science Paper highlights how ElectroHemp Phytoremediation Rafts can be used as Biofilters to clean pollution from water sources.
Acacia nilotica bark serves as an adsorbent of toxic metals. Bark (1 g) when added to 100 ml of aqueous solution containing 10 mg ml-1 metal solution exhibited different metal adsorption values for different metals. The order of metal adsorption being Cr > Ni > Cu > Cd> As > Pb. A similar trend of metal adsorption was observed when the bark is reused (1strecycle) Cr > Ni > Cu > Cd > Pb and also in the column-sorption. In order to verify the metal removal property of A. nilotica bark, toxicity bioassay with Salix viminalis stem cuttings in hydroponic system augmented with Cd, Cr and Pb together with A. nilotica bark powder was carried out. The results of toxicity bioassay confirmed the metal adsorption property of the bark powder. The functions of toxicity studies include leaf area, root length and number of new root primordia produced per stump. The leaf area, root length and number of new root primordia increased considerably in the presence of A. nilotica bark. The order of metal toxicity for leaf area and new root primordial is Cd > Cr > Pb. However, for root length the order of metal toxicity is Cr > Cd > Pb. The metal budgets of the leaf and root confirmed that the bark powder had adsorbed substantial amount of toxic metals and thus, alleviates the toxicity imposed by the various tested elements (Prasad et al. 2001).
Quercus ilex L. phytomass from stem, leaf and root as adsorbent of chromium, nickel, copper, cadmium and lead at ambient temperature was investigated. The metal uptake capacity of the root for different metals was found to be in the order of: Ni > Cd > Pb > Cu > Cr; stem Ni > Pb> Cu > Cd > Cr and leaf Ni > Cd > Cu > Pb > Cr. The highest amount adsorbed was Ni (root > leaf > stem). Data from this laboratory demonstrated that Ni is mostly sequestered in the roots where concentrations can be as high as 7.30 nmol/g dry weight, when one year old seedlings were treated with Ni (2000 mg/l) in pot culture experiments, compared to 0.13 nmol/g dry weight, in the control. This proves that the root biomass of Q. ilex has the capacity for complexing Ni. Chromium exhibited the least adsorption values for all the three types of phytomass compared to other metals. The trend of adsorption of the phytomass was similar for nickel and cadmium i.e. root > leaf > stem. Desorption with 10 mM Na2 EDTA was effective (55-90%). Hence, there exists the possibility of recycling the phytomass. The biosorption results of recycled phytomass suggests, that the selected adsorbents are reusable (Prasad and Freitas, 2000).
 |
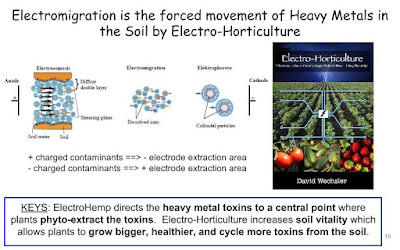
| Phytoremediation Raft Infographic- Plants cycle water toxins when grown on Rafts |
a wide variety of agricultural and forestry by products have been used as biosorbents of toxic metals in a bid to develop biofilters for specific applications Electronic Journal of Biotechnology:
A floating phytoremediation raft constructed of: waste tea leaves, Pinus pinaster bark, Olea europea, Acacia nilotica bark. Which has these plant examples growing on it: Kenaf, Water Lettuce, Alligator Weed create a combination of Natural Solutions in the detoxification of Lead (Pb) from water. Scotty, ElectroHempPhytoremediation Science Paper link
- i) Cotton - Hg; Groundnut skins - Cu;
- Tree Bark (Pinus, Acacia etc.) - variety of metals;
- Agrowaste - variery of metals;
- waste tea leaves - Pb, Cd, and Zn;
- Pinus radiata -U;
- Apple waste -Variety of metals;
- Cellulose - Variety of metals; Rice hulls - Variety of metals;
- Exhausted coffee grounds - Hg;
- Pinus pinaster bark - Zn, Cu, Pb. Saw mill dust (wood waste)- Cr;
- Freshwater green algae - variety of metals;
- Marine algae- Pb, Ni;
- ii) Sphagnum (moss peat) - Cr(VI);
- iii) Immobilized Aspergillus niger, A. oryzae - Cd, Cu, Pb, and Ni ;
- Olive mill waste Olea europea Cr, Ni, Pb, Cd, and Zn, Cu and Ni;
- Streptomyces rimosus (bacteria);
- Saccharomyces cerevisiae (yeast);
- Penicillium chrysogenum (fungi), Fuscus vesiculosus and Ascophyllum nodosum (marine algae) Zn, Cu andNi; Phanerochaete chrysosporium, P. versicolar - Pb, Ni, Cr, Cd, Cu; Pinus radiata - U;
- Immobilized Pseudomonas putida 5-X and Aspergillus niger, Mucor rouxxi - Cu;
- Actionomycetes, Aspergillus niger, A.oryzae, Rhizopus arrhizus, R. nigricans- Cd; Rhizopus arrhizus - Cr(VI), Pb; Rhizopus nigricans, Phanarochaete chrysogenum -Pb; Aspergillus niger and Rhizopus arrhizus - Ni
Acacia nilotica bark serves as an adsorbent of toxic metals. Bark (1 g) when added to 100 ml of aqueous solution containing 10 mg ml-1 metal solution exhibited different metal adsorption values for different metals. The order of metal adsorption being Cr > Ni > Cu > Cd> As > Pb. A similar trend of metal adsorption was observed when the bark is reused (1strecycle) Cr > Ni > Cu > Cd > Pb and also in the column-sorption. In order to verify the metal removal property of A. nilotica bark, toxicity bioassay with Salix viminalis stem cuttings in hydroponic system augmented with Cd, Cr and Pb together with A. nilotica bark powder was carried out. The results of toxicity bioassay confirmed the metal adsorption property of the bark powder. The functions of toxicity studies include leaf area, root length and number of new root primordia produced per stump. The leaf area, root length and number of new root primordia increased considerably in the presence of A. nilotica bark. The order of metal toxicity for leaf area and new root primordial is Cd > Cr > Pb. However, for root length the order of metal toxicity is Cr > Cd > Pb. The metal budgets of the leaf and root confirmed that the bark powder had adsorbed substantial amount of toxic metals and thus, alleviates the toxicity imposed by the various tested elements (Prasad et al. 2001).
Quercus ilex L. phytomass from stem, leaf and root as adsorbent of chromium, nickel, copper, cadmium and lead at ambient temperature was investigated. The metal uptake capacity of the root for different metals was found to be in the order of: Ni > Cd > Pb > Cu > Cr; stem Ni > Pb> Cu > Cd > Cr and leaf Ni > Cd > Cu > Pb > Cr. The highest amount adsorbed was Ni (root > leaf > stem). Data from this laboratory demonstrated that Ni is mostly sequestered in the roots where concentrations can be as high as 7.30 nmol/g dry weight, when one year old seedlings were treated with Ni (2000 mg/l) in pot culture experiments, compared to 0.13 nmol/g dry weight, in the control. This proves that the root biomass of Q. ilex has the capacity for complexing Ni. Chromium exhibited the least adsorption values for all the three types of phytomass compared to other metals. The trend of adsorption of the phytomass was similar for nickel and cadmium i.e. root > leaf > stem. Desorption with 10 mM Na2 EDTA was effective (55-90%). Hence, there exists the possibility of recycling the phytomass. The biosorption results of recycled phytomass suggests, that the selected adsorbents are reusable (Prasad and Freitas, 2000).








No comments:
Post a Comment