Coupled Electro-kinetic Remediation and Phytoremediation of Metal(loid)Contaminated Soils
Xinyu Mao 1,2,
Fengxiang X. Han 2
*,
Xiaohou Shao 1
and
Yi Su 3
1-College of Water Conservancy and Hydropower Engineering, Hohai University, China
2-Department of Chemistry and Biochemistry, Jackson State University, USA
3-Department of Chemistry and Biochemistry, Texas A&M University-Texarkana, USA
Coupled Electro-kinetic Remediation and Phytoremediation of Metal(loid) Contaminated Soils. J
Bioremed Biodeg 6: e163. doi:10.4172/2155-6199.1000e163
Volume 6 • Issue 2 • 1000e163
J Bioremed Biodeg
ISSN: 2155-6199 JBRBD, an open access journal
Soil contamination with heavy metals and metalloids has become
a serious environmental problem with rapid industrialization and
urbanization [1-3]. Generally, the contamination is resulted from
anthropogenic activities such as mining, domestic waste discharge,
agricultural production and industrial activities. Heavy metals and
metalloids such as Cd, Pb, Cr, Cu, Hg, Cs, Se, Zn and As enter the
food chain and have adverse effects on human health [1-3]. As the
increasing concern on the environmental risk, numerous remediation
technologies have been developed while very few methods had been
proved to be efficient for cleaning up of heavy metal(loid)s due to their
characteristics of persistence and non-degradation in contaminated
sites [1,4-10].
Phytoremediation is a cost-effective and environmental-friendly
remediation technology for the remediation of heavy metal(loid)d in
soils [4-6]. It promotes water and soil conservation as well as microbial
activity improvement. Thus it has proved to be efficient for large
area treatment with low pollutant concentration. However, several
limitations such as the remediation period, the climatic condition, the
root depth, the biomass production and the variety of the contaminants
are existed in actual applications [11]. Therefore, selection of plants with
high accumulation capacity of contaminants and new technologies for
increasing soil heavy metal(loid) bioavailability should be developed.
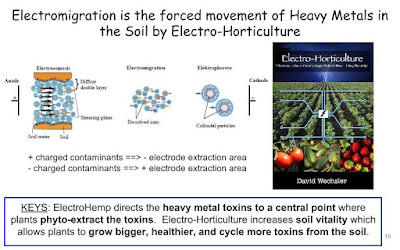
Electro-kinetic remediation (EKR) which involves a low intensity
electric field has been proposed to enhance phytoremediation. It
produces conditions to solubilize metal(loid)s in soils based on the
combined mechanisms of electro-osmosis, electromigration and
electrophoresis [12]. As a consequence of driving force generated by
the passage of current, metal(loid) ions and metal complex migrated
from anodes to cathodes could be easily absorbed by plants. Since soil
pH polarizations caused by electrolytic decomposition at electrodes,
control of soil pH and some key electronic parameters are important
for the remediation efficiency [13].
This paper reviews the current development on coupled electrokinetic
phytoremediation (EK-phytoremediation) technology,
including the selection principles of plants for the technology and
interactions between heavy metal(loid) input and their bioavailability
in soils. Furthermore, assisted amendments and key electronic
parameters for the improvement of soil physical-chemical properties
and plants remediation effects are also discussed in the paper.
Coupled EK-phytoremediation Technology
Generally, coupled EK-phytoremediation techniques contain
the application of a low intensity electric field adjoined to growing
plants in contaminated soil. Significant variables which can affect the
technology include the electric field intensity, use of AC or DC current,
mode of voltage application, electrodes configurations and facilitating
amendments. Lemstrom first applied electric fields on growing plants
and found treated plants were greener and experienced an increase
in yield [14] The removal of contaminants by plants in coupled
EK-phytoremediation technology are enhanced by increasing the
bioavailability of the contaminants through the effect of electric field.
Increased soluble heavy metals are drive to the plant roots which might
bring stress condition for the plants. Therefore, hyperaccumulators
are the best choice for the coupled remediation [15]. It has been
proposed sequential use of phytoremediation after EKR is beneficial
for the cleanup of residue contaminants and recovery of soil properties
damage caused by EKR [16]. However, coupled EK-phytoremediation
technology has been proved to be more efficient and effective than the
sequential use of these technologies.
Plant Selection
Phytostabilization and phytoextraction are often used for
the cleaning up of contaminants. Compared to phytoextraction,
phytostablization is not a permanent strategy since the contaminants
remain in environment. Therefore, phytoextraction becomes the most
promising and commercial application for heavy metal(loid) cleaning
up in soils. Plants that suitable for phytoextraction possibly should
have the features with high growth rate, high resistance to pathogens
and pests, highly developed root system, large biomass production of
aerial parts, high tolerance to trace metal(loid), great accumulation and
translocation ability and good environment adaptability [17].
Metals accumulation and biomass are two critical factors
for determination of plant species for phytoextraction purpose.
Hyperaccumulators often with comparatively low over-ground
biomass, accumulate greater amount of target metals. Other plants
such as Indian mustard accumulates less metal but produces more
over-ground biomass so that the overall accumulation is comparable
to that of hyperaccumulators. Hyperaccumulation and hypertolerance
are more important than high biomass in phytoremediation. In
addition, compared with nonaccumulators, hyperaccumulators with
high accumulation of metals in small volume of biomass are easier and
more economic to operate for either metals recovery or safe disposal
[18]. Plants with multiple harvests in a single growth period have great
potentials for phytoextraction. In addition, due to the high growth rate,
great adaptability and high biomass, grasses are more favorable than
shrubs and trees in phytoremediation [19].
Bioavailability of Heavy Metals in Soils
Bioavailability of trace metal(loid)s in soil is a determining factor
which influencing the efficiency of phytoextraction. Only a part of metal
in soil is bioavailable for plants uptake. Processes like precipitation
or strong binding to soil particles make a large part of soil metals
insoluble and unavailable for plants extraction. Metal(loid)s in soil
are divided into three categories according to their bioavailability:
readily bioavailable (Cd, Ni, Zn, As, Se, Cu); moderately bioavailable
(Co, Mn, Fe) and least bioavailable (Pb, Cr, U) [20] Low bioavailability
constrains the phytoextraction effects of heavy metals while the changes
of soil physical-chemical properties may enhance the bioavailability
and mobility of metals. Secretion of H+ ions by roots will be able to
demobilize more metals around rhizosphere. Moreover, activities
of rhizospheric microbes significantly increase labile metals in soil.
Except enhanced in natural phytoextraction, bioavailability of heavy
metals in soil can also be increased by adding chelating agents such
as EDTA, ammonium sulfate, critic acid and elemental sulfur et al. in
induced phytoextraction [9].
Key Electronic Parameters for EK-phytoremediation
Technology
Significant electronic parameters are electric field intensity,
use of AC/DC current, mode of voltage application and electrodes
configurations. Electric field intensity has a determining influence on
the effectiveness of EK-phytoremediation. Low voltage was beneficial
for Indian mustard growth while the biomass production was decreased
as the increase of voltage [21]. However, the increasing bioavailability
of heavy metals at elevated voltage and the negative effects of the voltage
on the plant development were comprised at an intermediate voltage of
2V with the best metal removal and accumulation on plant tissues [21].
Soil pH was reported to decrease from initial pH 6.5 to 3 near the
anode and increased to 8 near the cathode with application of DC
electric field with potato [22]. Heavy metals had a migration from
anode to cathode and an accumulation in the middle of the pot where
the pH was 5. On the contrary, no transportation and soil pH change
was observed with application of AC electric current. Moreover,
potato had 72% increase whereas 27% decline in biomass production
under AC and DC electric field, respectively. Overall, test using AC
electric field showed higher metals accumulation in both plant roots
and shoots than the control test and the DC test [22]. Soil pH changes
which resulted from the electrolysis of water were induced by the use
of continuous DC electric field. In order to avoid the negative effects,
switching the polarity of the DC electric field every 3 h eliminated pH
changes and comparable phytoremediation efficiency between the DC
and AC tests were achieved [23].
The effectiveness of coupled EK-phytoremediation was influenced
by electrode configurations. An vertical DC electric field or several
electrode arrangements were extended with phytoremediation depth,
preventing leaching of Pb [24,25]. The configuration with the cathode
in the center surrounded with anodes showed greater potential to
metals accumulation [25] Recently a 2D electrode configuration with
cathode were placed on the surface of the soil and anodes was vertically
installed in four corners of a rectangular chamber. The results showed
enhancement in both metal accumulation in roots and shoots and
metal translocation towards the shoots [26].
Amendments for EK-phytoremediation
In order to improve metals bioavailability, control soil pH with the
favorable range and facilitate plants growth, amendments are added,
including chelating or complexing agents, organic amendments and
fertilizers etc. Chelants enhance EK-phytoremediation by forming
strong water-soluble complex which desorbs metal(loid)s from soil
particle surface. EDTA is most frequently used chelant and has been
proved to be effective on mobilization of metals like Cr, Fe, Cu, Pb
and Zn [9]. The factors included metals species, metal/chelate ratio,
presence of competing cations and soil pH et al. In addition, EDTA
showed some phytotoxicicty to plants [9]. Complexing agents such as
I−, Cl−, NH4
+ may form soluble complexes with metal ions. Ammonium
thiosulphate could result in the solubilization of Hg enhancing
accumulation in plant roots and shoots [27]. Furthermore, acetic
acid and lactic acid is used to neutralize the electrolysis product at the
cathode and keep the electrolyte pH within a certain range. Ammonium
acetate was beneficial for increasing Cu solubility and removal rate [28]
Organic amendments such as sewage sludge and green waste composts
improve plant growth by enhancing the physicochemical and biological
conditions of soils. They also directly or indirectly alter the distribution
and availability of soil metals. Several reports revealed that amended
compost increased As mobility due to the competing effect of DOC
and soluble P component with As for sorption sites. Moreno-Jimenez
et al. discovered the mobilization of As, Cu and Se in flooded soils
after the application of olive mill waste compost. In contrast, reports
also indicated that the bioavailability of Pb was decreased when added
compost [27]. Clemente et al. [29] found an increase in mobility of As
and Sb after the two years application of compost mulch in soil which
enhanced the uptake by lettuce and sunflower [29].
Conclusion
The coupled EK-phytoremediation technology is promising for the
clean-up of heavy metal(loid)s in contaminated soils. More research
is necessary for its practical design and application before applying at
field scale. The research directions are suggested in following aspects
such as: determine the distribution, translocation and environmental
risks of heavy metal(loid)s and their influence on plants metal(loid)
s accumulation; test and select the hyper accumulators which possess
remarkable metal(loid)s accumulation ability for this coupled
technology; test the remediation efficiency of the coupled technology
in sites with both organic and inorganic contaminants; try to apply
assisted amendments of natural or biodegradable products; elucidate
the mechanisms and influence of electronic parameters on metabolism
and growth of plant as well as uptake and translocation of metal(loid)s.
Acknowledgement
This research was supported by U.S. Nuclear Regulatory Commission (NRC–
HQ-12-G-38-0038), NOAA-ECSC grant (NA11SEC4810001), NIH-RCMI grant
(G12MD007581), the Jiangsu Scientific Research Innovation Program of Ordinary
Higher Education Graduate (China) (KYZZ0156), the Fundamental Research
Funds for the Central Universities (China) (2014B00114).
References
1. Han FX, Arieh Singer (2007) Biogeochemistry of Trace Elements in Arid
Environments. Springer.
2. Han FX, Banin A, Su Y, Monts DL, Plodinec MJ, et al. (2002) Industrial age
anthropogenic inputs of heavy metals into the pedosphere. Naturwissenschaften
89: 497-504.
3. Han FX, Su Y, Monts DL, Plodinec MJ, Banin A, et al. (2003) Assessment of global
industrial-age anthropogenic arsenic contamination. Naturwissenschaften 90:
395-401.
4. Shiyab S, Chen J, Han FX, Monts DL, Matta FB, et al. (2009) Phytotoxicity
of mercury in Indian mustard (Brassica juncea L.). Ecotoxicol Environ Saf 72:
619-625.
5. Chen J, Shiyab S, Han FX, Monts DL, Waggoner CA, et al. (2009)
Bioaccumulation and physiological effects of mercury in Pteris vittata and
Nephrolepis exaltata. Ecotoxicol 18 (1): 110-121.
6. Su Y, Han FX, Chen J, Sridhar BBM, Monts DL (2008) Phytoextraction and
accumulation of mercury in three plant species: Indian mustard (Brassica
juncea), Beard grass (Polypogon monospeliensis), and Chinese brake fern
(Pteris vittata). Int J Phytorem 10: 547-560.
7. Su Y, Sridhar BB, Han FX, Diehl SV, Monts DL (2007) Effects of bioaccumulation
of Cs and Sr natural isotopes on foliar structure and plant spectral reflectance
of Indian mustard (Brassica Juncea). Water Air Soil Pollut 180: 65-74.
8. Su Y, Han FX, Sridhar BBM, Monts DL (2005) Phytotoxicity and
phytoaccumulation of trivalent and hexavalent chromium in brake fern. Environ
Toxicol Chem 24: 2019-2026.
9. Han FX, Su Y, Monts DL, Sridhar BBM (2004) Distribution, transformation and
bioavailability of trivalent and hexavalent chromium in contaminated soil. Plant
Soil 265: 243-252.
10. Han FX, Sridhar BBM, Monts DL, Su Y (2004) Phytoavailability and toxicity of
trivalent and hexavalent chromium to Brassica juncea L. Czern. New Phytol
162: 489-499.
11. Rungwa S, Arpa G, Sakulas H, Harakuwe A, Timie D (2013) Phytoremediationan
eco-friendly and sustainable method of heavy metal removal from closed
mine environments in Papua New Guinea. Procedia Earth Planet Sci 6: 269-
277.
12. Cameselle C, Reddy KR (2012) Development and enhancement of electroosmotic
flow for the removal of contaminants from soils. Electrochim Acta 86:
10-22.
13. Reddy KR, Cameselle C (2009) Electrochemical remediation technologies for
polluted soils, sediments and groundwater, John Wiley & Sons, Hoboken, USA.
14. Lemstrom S (1904) Electricity in agriculture and horticulture. The Electrician
Printing & Publishing, London, UK.
15. Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of
metal-contaminated land. A review. Environ Chem Lett 8: 1-17.
16. Wan QF, Deng DC, Bai Y, Xia CQ (2012) Phytoremediation and electrokinetic
remediation of uranium contaminated soils: a review. He-Huaxue yu Fangshe
Huaxue/ J Nucl Radiochem 34: 148-156.
17. Pulford ID, Watson C (2003) Phytoremediation of heavy metal-contaminated
land by trees-a review. Environ Int 29: 529-540.
18. Chaney RL, Malik KM, Li YM, Brown SL, Brewer EP, et al. (1997)
Phytoremediation of soil metals. Curr Opin Biotechnol 8: 279-284.
19. Ebbs SD, Kochian LV (1997) Toxicity of zinc and copper to Brassica species:
implications for phytoremediation. J Environ Qual 26: 776-781.
20. Zhang C, Yu ZG, Zeng GM, Jiang M, Yang ZZ, et al. (2014) Effects of sediment
geochemical properties on heavy metal bioavailability. Environ Int 73: 270-281.
21. Cang L, Wang QY, Zhou DM, Xu H (2011) Effects of electrokinetic-assisted
phytoremediation of a multiple-metal contaminated soil on soil metal
bioavailability and uptake by Indian mustard. Sep Purif Technol. 79: 246-253.
22. Aboughalma H, Bi R, Schlaak M (2008) Electrokinetic enhancement on
phytoremediation in Zn, Pb, Cu and Cd contaminated soil using potato plants.
J Environ Sci Health Part A 43: 926-933.
23. Bi R, Schlaak M, Siefert E, Lord R, Connolly H (2011) Influence of electrical
fields (AC and DC) on phytoremediation of metal polluted soils with rapeseed
(Brassica napus) and tobacco (Nicotiana tabacum). Chemosphere 83: 318-
326.
24. Zhou DM, Chen HF, Cang L, Wang YJ (2007) Ryegrass uptake of soil Cu/Zn
induced by EDTA/EDDS together with a vertical direct-current electrical field.
Chemosphere 67: 1671-1676.
25. Hodko D, Hyfte JV, Denvir A, Magnuson JW (2000) Methods for enhancing
phytoextraction of contaminants from porous media using electrokinetic
phenomena.
26. Putra RS, Ohkawa Y, Tanaka S (2013) Application of EAPR system on the
removal of lead from sandy soil and uptake by Kentucky bluegrass (Poa
pratensis L.). Sep Purif Technol. 102: 34-42.
27. Moreno FN, Anderson CWN, Stewart RB, Robinson BH, Ghomshei M, et
al. (2005) Induced plant uptake and transport of mercury in the presence of
sulphur-containing ligands and humic acids. New Phytol 166: 445-454.
28. Chen JL, Yang SF, Wu CC, Ton S (2011) Effect of ammonia as a complexing
agent on electrokinetic remediation of copper-contaminated soil, Sep Purif
Technol 79: 157-163.
29. Clemente R, Hartley W, Riby P, Dickinson NM, Lepp NW (2010) Trace element
mobility in a contaminated soil two years after field-amendment with a green
waste compost mulch. Environ Pollut. 158: 1644-1651.