Xinyu Mao 1,2,
Fengxiang X. Han 2 *,
Xiaohou Shao 1 and
Yi Su 3
1-College of Water Conservancy and Hydropower Engineering, Hohai University, China
2-Department of Chemistry and Biochemistry, Jackson State University, USA
3-Department of Chemistry and Biochemistry, Texas A&M University-Texarkana, USA
Coupled Electro-kinetic Remediation and Phytoremediation of Metal(loid) Contaminated Soils. J Bioremed Biodeg 6: e163. doi:10.4172/2155-6199.1000e163 Volume 6 • Issue 2 • 1000e163 J Bioremed Biodeg ISSN: 2155-6199 JBRBD, an open access journal
Soil contamination with heavy metals and metalloids has become a serious environmental problem with rapid industrialization and urbanization [1-3]. Generally, the contamination is resulted from anthropogenic activities such as mining, domestic waste discharge, agricultural production and industrial activities. Heavy metals and metalloids such as Cd, Pb, Cr, Cu, Hg, Cs, Se, Zn and As enter the food chain and have adverse effects on human health [1-3]. As the increasing concern on the environmental risk, numerous remediation technologies have been developed while very few methods had been proved to be efficient for cleaning up of heavy metal(loid)s due to their characteristics of persistence and non-degradation in contaminated sites [1,4-10].
Phytoremediation is a cost-effective and environmental-friendly remediation technology for the remediation of heavy metal(loid)d in soils [4-6]. It promotes water and soil conservation as well as microbial activity improvement. Thus it has proved to be efficient for large area treatment with low pollutant concentration. However, several limitations such as the remediation period, the climatic condition, the root depth, the biomass production and the variety of the contaminants are existed in actual applications [11]. Therefore, selection of plants with high accumulation capacity of contaminants and new technologies for increasing soil heavy metal(loid) bioavailability should be developed.
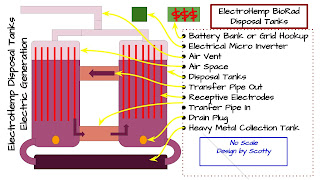
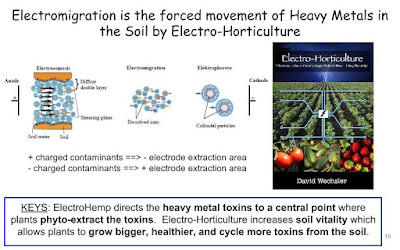
Electro-kinetic remediation (EKR) which involves a low intensity electric field has been proposed to enhance phytoremediation. It produces conditions to solubilize metal(loid)s in soils based on the combined mechanisms of electro-osmosis, electromigration and electrophoresis [12]. As a consequence of driving force generated by the passage of current, metal(loid) ions and metal complex migrated from anodes to cathodes could be easily absorbed by plants. Since soil pH polarizations caused by electrolytic decomposition at electrodes, control of soil pH and some key electronic parameters are important for the remediation efficiency [13].
This paper reviews the current development on coupled electrokinetic phytoremediation (EK-phytoremediation) technology, including the selection principles of plants for the technology and interactions between heavy metal(loid) input and their bioavailability in soils. Furthermore, assisted amendments and key electronic parameters for the improvement of soil physical-chemical properties and plants remediation effects are also discussed in the paper.
Coupled EK-phytoremediation Technology
Generally, coupled EK-phytoremediation techniques contain the application of a low intensity electric field adjoined to growing plants in contaminated soil. Significant variables which can affect the technology include the electric field intensity, use of AC or DC current, mode of voltage application, electrodes configurations and facilitating amendments. Lemstrom first applied electric fields on growing plants and found treated plants were greener and experienced an increase in yield [14] The removal of contaminants by plants in coupled EK-phytoremediation technology are enhanced by increasing the bioavailability of the contaminants through the effect of electric field. Increased soluble heavy metals are drive to the plant roots which might bring stress condition for the plants. Therefore, hyperaccumulators are the best choice for the coupled remediation [15]. It has been proposed sequential use of phytoremediation after EKR is beneficial for the cleanup of residue contaminants and recovery of soil properties damage caused by EKR [16]. However, coupled EK-phytoremediation technology has been proved to be more efficient and effective than the sequential use of these technologies.
Plant Selection
Metals accumulation and biomass are two critical factors for determination of plant species for phytoextraction purpose. Hyperaccumulators often with comparatively low over-ground biomass, accumulate greater amount of target metals. Other plants such as Indian mustard accumulates less metal but produces more over-ground biomass so that the overall accumulation is comparable to that of hyperaccumulators. Hyperaccumulation and hypertolerance are more important than high biomass in phytoremediation. In addition, compared with nonaccumulators, hyperaccumulators with high accumulation of metals in small volume of biomass are easier and more economic to operate for either metals recovery or safe disposal [18]. Plants with multiple harvests in a single growth period have great potentials for phytoextraction. In addition, due to the high growth rate, great adaptability and high biomass, grasses are more favorable than shrubs and trees in phytoremediation [19].
Bioavailability of Heavy Metals in Soils
Key Electronic Parameters for EK-phytoremediation
Technology
Significant electronic parameters are electric field intensity, use of AC/DC current, mode of voltage application and electrodes configurations. Electric field intensity has a determining influence on the effectiveness of EK-phytoremediation. Low voltage was beneficial for Indian mustard growth while the biomass production was decreased as the increase of voltage [21]. However, the increasing bioavailability of heavy metals at elevated voltage and the negative effects of the voltage on the plant development were comprised at an intermediate voltage of 2V with the best metal removal and accumulation on plant tissues [21].
Soil pH was reported to decrease from initial pH 6.5 to 3 near the anode and increased to 8 near the cathode with application of DC electric field with potato [22]. Heavy metals had a migration from anode to cathode and an accumulation in the middle of the pot where the pH was 5. On the contrary, no transportation and soil pH change was observed with application of AC electric current. Moreover, potato had 72% increase whereas 27% decline in biomass production under AC and DC electric field, respectively. Overall, test using AC electric field showed higher metals accumulation in both plant roots and shoots than the control test and the DC test [22]. Soil pH changes which resulted from the electrolysis of water were induced by the use of continuous DC electric field. In order to avoid the negative effects, switching the polarity of the DC electric field every 3 h eliminated pH changes and comparable phytoremediation efficiency between the DC and AC tests were achieved [23].
The effectiveness of coupled EK-phytoremediation was influenced by electrode configurations. An vertical DC electric field or several electrode arrangements were extended with phytoremediation depth, preventing leaching of Pb [24,25]. The configuration with the cathode in the center surrounded with anodes showed greater potential to metals accumulation [25] Recently a 2D electrode configuration with cathode were placed on the surface of the soil and anodes was vertically installed in four corners of a rectangular chamber. The results showed enhancement in both metal accumulation in roots and shoots and metal translocation towards the shoots [26].
Amendments for EK-phytoremediation
In order to improve metals bioavailability, control soil pH with the favorable range and facilitate plants growth, amendments are added, including chelating or complexing agents, organic amendments and fertilizers etc. Chelants enhance EK-phytoremediation by forming strong water-soluble complex which desorbs metal(loid)s from soil particle surface. EDTA is most frequently used chelant and has been proved to be effective on mobilization of metals like Cr, Fe, Cu, Pb and Zn [9]. The factors included metals species, metal/chelate ratio, presence of competing cations and soil pH et al. In addition, EDTA showed some phytotoxicicty to plants [9]. Complexing agents such as I−, Cl−, NH4 + may form soluble complexes with metal ions. Ammonium thiosulphate could result in the solubilization of Hg enhancing accumulation in plant roots and shoots [27]. Furthermore, acetic acid and lactic acid is used to neutralize the electrolysis product at the cathode and keep the electrolyte pH within a certain range. Ammonium acetate was beneficial for increasing Cu solubility and removal rate [28] Organic amendments such as sewage sludge and green waste composts improve plant growth by enhancing the physicochemical and biological conditions of soils. They also directly or indirectly alter the distribution and availability of soil metals. Several reports revealed that amended compost increased As mobility due to the competing effect of DOC and soluble P component with As for sorption sites. Moreno-Jimenez et al. discovered the mobilization of As, Cu and Se in flooded soils after the application of olive mill waste compost. In contrast, reports also indicated that the bioavailability of Pb was decreased when added compost [27]. Clemente et al. [29] found an increase in mobility of As and Sb after the two years application of compost mulch in soil which enhanced the uptake by lettuce and sunflower [29].
Conclusion
The coupled EK-phytoremediation technology is promising for the clean-up of heavy metal(loid)s in contaminated soils. More research is necessary for its practical design and application before applying at field scale. The research directions are suggested in following aspects such as: determine the distribution, translocation and environmental risks of heavy metal(loid)s and their influence on plants metal(loid) s accumulation; test and select the hyper accumulators which possess remarkable metal(loid)s accumulation ability for this coupled technology; test the remediation efficiency of the coupled technology in sites with both organic and inorganic contaminants; try to apply assisted amendments of natural or biodegradable products; elucidate the mechanisms and influence of electronic parameters on metabolism and growth of plant as well as uptake and translocation of metal(loid)s.
Acknowledgement
This research was supported by U.S. Nuclear Regulatory Commission (NRC– HQ-12-G-38-0038), NOAA-ECSC grant (NA11SEC4810001), NIH-RCMI grant (G12MD007581), the Jiangsu Scientific Research Innovation Program of Ordinary Higher Education Graduate (China) (KYZZ0156), the Fundamental Research Funds for the Central Universities (China) (2014B00114).
References 1. Han FX, Arieh Singer (2007) Biogeochemistry of Trace Elements in Arid Environments. Springer.
2. Han FX, Banin A, Su Y, Monts DL, Plodinec MJ, et al. (2002) Industrial age anthropogenic inputs of heavy metals into the pedosphere. Naturwissenschaften 89: 497-504.
3. Han FX, Su Y, Monts DL, Plodinec MJ, Banin A, et al. (2003) Assessment of global industrial-age anthropogenic arsenic contamination. Naturwissenschaften 90: 395-401.
4. Shiyab S, Chen J, Han FX, Monts DL, Matta FB, et al. (2009) Phytotoxicity of mercury in Indian mustard (Brassica juncea L.). Ecotoxicol Environ Saf 72: 619-625.
5. Chen J, Shiyab S, Han FX, Monts DL, Waggoner CA, et al. (2009) Bioaccumulation and physiological effects of mercury in Pteris vittata and Nephrolepis exaltata. Ecotoxicol 18 (1): 110-121.
6. Su Y, Han FX, Chen J, Sridhar BBM, Monts DL (2008) Phytoextraction and accumulation of mercury in three plant species: Indian mustard (Brassica juncea), Beard grass (Polypogon monospeliensis), and Chinese brake fern (Pteris vittata). Int J Phytorem 10: 547-560.
7. Su Y, Sridhar BB, Han FX, Diehl SV, Monts DL (2007) Effects of bioaccumulation of Cs and Sr natural isotopes on foliar structure and plant spectral reflectance of Indian mustard (Brassica Juncea). Water Air Soil Pollut 180: 65-74.
8. Su Y, Han FX, Sridhar BBM, Monts DL (2005) Phytotoxicity and phytoaccumulation of trivalent and hexavalent chromium in brake fern. Environ Toxicol Chem 24: 2019-2026.
9. Han FX, Su Y, Monts DL, Sridhar BBM (2004) Distribution, transformation and bioavailability of trivalent and hexavalent chromium in contaminated soil. Plant Soil 265: 243-252.
10. Han FX, Sridhar BBM, Monts DL, Su Y (2004) Phytoavailability and toxicity of trivalent and hexavalent chromium to Brassica juncea L. Czern. New Phytol 162: 489-499.
11. Rungwa S, Arpa G, Sakulas H, Harakuwe A, Timie D (2013) Phytoremediationan eco-friendly and sustainable method of heavy metal removal from closed mine environments in Papua New Guinea. Procedia Earth Planet Sci 6: 269- 277.
12. Cameselle C, Reddy KR (2012) Development and enhancement of electroosmotic flow for the removal of contaminants from soils. Electrochim Acta 86: 10-22.
13. Reddy KR, Cameselle C (2009) Electrochemical remediation technologies for polluted soils, sediments and groundwater, John Wiley & Sons, Hoboken, USA.
14. Lemstrom S (1904) Electricity in agriculture and horticulture. The Electrician Printing & Publishing, London, UK.
15. Vamerali T, Bandiera M, Mosca G (2010) Field crops for phytoremediation of metal-contaminated land. A review. Environ Chem Lett 8: 1-17.
16. Wan QF, Deng DC, Bai Y, Xia CQ (2012) Phytoremediation and electrokinetic remediation of uranium contaminated soils: a review. He-Huaxue yu Fangshe Huaxue/ J Nucl Radiochem 34: 148-156.
17. Pulford ID, Watson C (2003) Phytoremediation of heavy metal-contaminated land by trees-a review. Environ Int 29: 529-540.
18. Chaney RL, Malik KM, Li YM, Brown SL, Brewer EP, et al. (1997) Phytoremediation of soil metals. Curr Opin Biotechnol 8: 279-284.
19. Ebbs SD, Kochian LV (1997) Toxicity of zinc and copper to Brassica species: implications for phytoremediation. J Environ Qual 26: 776-781.
20. Zhang C, Yu ZG, Zeng GM, Jiang M, Yang ZZ, et al. (2014) Effects of sediment geochemical properties on heavy metal bioavailability. Environ Int 73: 270-281.
21. Cang L, Wang QY, Zhou DM, Xu H (2011) Effects of electrokinetic-assisted phytoremediation of a multiple-metal contaminated soil on soil metal bioavailability and uptake by Indian mustard. Sep Purif Technol. 79: 246-253.
22. Aboughalma H, Bi R, Schlaak M (2008) Electrokinetic enhancement on phytoremediation in Zn, Pb, Cu and Cd contaminated soil using potato plants. J Environ Sci Health Part A 43: 926-933.
23. Bi R, Schlaak M, Siefert E, Lord R, Connolly H (2011) Influence of electrical fields (AC and DC) on phytoremediation of metal polluted soils with rapeseed (Brassica napus) and tobacco (Nicotiana tabacum). Chemosphere 83: 318- 326.
24. Zhou DM, Chen HF, Cang L, Wang YJ (2007) Ryegrass uptake of soil Cu/Zn induced by EDTA/EDDS together with a vertical direct-current electrical field. Chemosphere 67: 1671-1676.
25. Hodko D, Hyfte JV, Denvir A, Magnuson JW (2000) Methods for enhancing phytoextraction of contaminants from porous media using electrokinetic phenomena.
26. Putra RS, Ohkawa Y, Tanaka S (2013) Application of EAPR system on the removal of lead from sandy soil and uptake by Kentucky bluegrass (Poa pratensis L.). Sep Purif Technol. 102: 34-42.
27. Moreno FN, Anderson CWN, Stewart RB, Robinson BH, Ghomshei M, et al. (2005) Induced plant uptake and transport of mercury in the presence of sulphur-containing ligands and humic acids. New Phytol 166: 445-454.
28. Chen JL, Yang SF, Wu CC, Ton S (2011) Effect of ammonia as a complexing agent on electrokinetic remediation of copper-contaminated soil, Sep Purif Technol 79: 157-163.
29. Clemente R, Hartley W, Riby P, Dickinson NM, Lepp NW (2010) Trace element mobility in a contaminated soil two years after field-amendment with a green waste compost mulch. Environ Pollut. 158: 1644-1651.